Protocatechualdehyde in Medicine: What We Know and What Lies Ahead
DOI:
https://doi.org/10.55578/amsr.2601.001Keywords:
Protocatechualdehyde (PubChem CID: 8768), Anti-oxidant, Anti-inflammatory, Traditional Chinese Medicine, Salvia miltiorrhizaAbstract
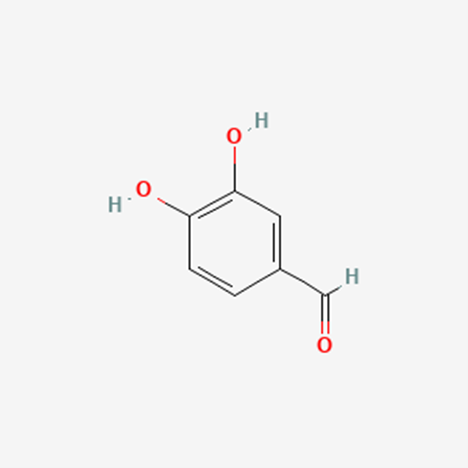
Background: Protocatechualdehyde (PCA) is a naturally-occurring phenolic aldehyde commonly found in many plants and plant-based products. PCA has many possible health benefits. It has shown promising anti-oxidant, anti-inflammatory, anti-bacterial and anti-carcinogenic traits.
Objective: In this study, we aim to explore PCA’s potential uses as a therapeutic agent and recommend future research directions.
Methods: PubMed was searched in November 2024. Every article containing “protocatechualdehyde” or “protocatechuic aldehyde” in title or abstract were looked into and relevant studies selected.
Results: PCA has the potential to be effective on a variety of disease. The top five most researched on conditions are: wound healing, atherosclerosis, myocardial ischemia, cerebrovascular disease, Parkinson’s disease. Articles mainly focus on cardiovascular, central nervous and urinary systems. Wound-sealant hydrogels containing PCA as a topical agent are an area of focus as well. anti-oxidant, anti-inflammatory, and anti-bacterial properties of PCA appear to give PCA its therapeutic potential. PCA can impact many intracellular pathways including Wnt/β-catenin signaling pathway and induce antiapoptotic proteins like B-cell lymphoma protein.
Discussion: In conclusion, studies regarding PCA’s therapeutic use have been diverse, but dispersed, and in a few instances contradictory. PCA can have a positive impact on a range of illnesses and conditions in vitro and in vivo. It appears that PCA owes its therapeutic features to its strong anti-oxidant, anti-inflammatory, and anti-bacterial effects. However, Current research lacks in showing PCA’s possible systemic effects and interaction with human body. PCA can become a potent pharmacological agent in the future, but still has a long journey ahead.
References
1. Information, N.C.f.B., PubChem Compound Summary for CID 8768, Protocatechualdehyde. 2024: https://pubchem.ncbi.nlm.nih.gov/compound/Protocatechualdehyde.
2. Zhang, S., et al., Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid Based Complement Alternat Med, 2021. 2021: p. 6139308.
3. Du, G.-H., et al., Protocatechualdehyde. Natural Small Molecule Drugs from Plants, 2018: p. 115-120.
4. Zhong, S., et al., Phellinus gilvus‑derived protocatechualdehyde induces G0/G1 phase arrest and apoptosis in murine B16‑F10 cells. Mol Med Rep, 2020. 21(3): p. 1107-1114.
5. Hu, D., et al., A Study and In vitro Evaluation of the Bioactive Compounds of Broad Bean Sprouts for the Treatment of Parkinson's Syndrome. Molecules, 2024. 29(21).
6. Rao, S., et al., Chemopreventive Potential of Cereal Polyphenols. Nutr Cancer, 2018. 70(6): p. 913-927.
7. Chang, Y.T., et al., Evaluation of the Therapeutic Effects of Protocatechuic Aldehyde in Diabetic Nephropathy. Toxins (Basel), 2021. 13(8).
8. Shi, X., et al., Protective effect of Gastrodia elata Blume in a Caenorhabditis elegans model of Alzheimer's disease based on network pharmacology. Biomed Rep, 2023. 18(5): p. 37.
9. Pei, J., et al., Protocatechuic aldehyde acts synergistically with dacarbazine to augment DNA double-strand breaks and promote apoptosis in cutaneous melanoma cells. BMC Complement Med Ther, 2023. 23(1): p. 111.
10. Wang, L., et al., Salvia miltiorrhiza: A Potential Red Light to the Development of Cardiovascular Diseases. Curr Pharm Des, 2017. 23(7): p. 1077-1097.
11. Li, Z.-m., S.-w. Xu, and P.-q. Liu, Salvia miltiorrhiza Burge (Danshen): a golden herbal medicine in cardiovascular therapeutics. Acta Pharmacologica Sinica, 2018. 39(5): p. 802-824.
12. Jia, Q., et al., Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine, 2019. 58: p. 152871.
13. Wu, X., et al., Protocatechuic aldehyde protects cardiomycoytes against ischemic injury via regulation of nuclear pyruvate kinase M2. Acta Pharm Sin B, 2021. 11(11): p. 3553-3566.
14. Ding, M., et al., Aqueous extract of Salvia miltiorrhiza attenuates increased endothelial permeability induced by tumor necrosis factor-alpha. Int Immunopharmacol, 2005. 5(11): p. 1641-51.
15. Ji, B., et al., Protocatechualdehyde restores endothelial dysfunction in streptozotocin-induced diabetic rats. Annals of Translational Medicine, 2021. 9(8).
16. Gulcin, İ. and S.H. Alwasel, DPPH Radical Scavenging Assay. Processes, 2023. 11(8): p. 2248.
17. Huang, S.-S., et al., Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. Journal of Ethnopharmacology, 2013. 147(2): p. 395-405.
18. Kim, K.-J., M.-A. Kim, and J.-H. Jung, Antitumor and antioxidant activity of protocatechualdehyde produced from Streptomyces lincolnensis M-20. Archives of pharmacal research, 2008. 31: p. 1572-1577.
19. Chang, Z.-Q., et al., In vitro antioxidant and anti-inflammatory activities of protocatechualdehyde isolated from Phellinus gilvus. Journal of nutritional science and vitaminology, 2011. 57(1): p. 118-122.
20. Chaouche, M., et al., Phytochemical study and antioxidant activities of the water-soluble aerial parts and isolated compounds of Thymus munbyanus subsp. ciliatus (Desf.) Greuter & Burdet. Turkish Journal of Pharmaceutical Sciences, 2021. 18(4): p. 430.
21. Shen, J., et al., Analysis of active components in Salvia miltiorrhiza injection based on vascular endothelial cells protection. Acta Pharmaceutica, 2014. 64(3): p. 325-334.
22. Zhong, Z., et al., Protocatechuic aldehyde mitigates hydrogen peroxide-triggered PC12 cell damage by down-regulating MEG3. Artificial Cells, Nanomedicine, and Biotechnology, 2020. 48(1): p. 602-609.
23. Gay, N.H., et al., Neuroprotective effects of phenolic and carboxylic acids on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Neurochemical research, 2018. 43: p. 619-636.
24. Wang, Y.-Q., G. Zhuang, and S.-J. Li, Multiple on-line screening and identification methods for hydroxyl radical scavengers in YuDanshen. Journal of Pharmaceutical and Biomedical Analysis, 2018. 156: p. 278-283.
25. Burnaz, N.A., M. Küçük, and Z. Akar, An on-line HPLC system for detection of antioxidant compounds in some plant extracts by comparing three different methods. Journal of Chromatography B, 2017. 1052: p. 66-72.
26. Li, Y., et al., Study on the anti-inflammatory effects of Callicarpa nudiflora based on the spectrum–effect relationship. Frontiers in Pharmacology, 2022. 12: p. 806808.
27. Ye, T., et al., Effect of Danshen on TLR2-triggered inflammation in macrophages. Phytomedicine, 2020. 70: p. 153228.
28. Jiang, X., et al., Bioactivity-integrated UPLC/Q-TOF–MS of Danhong injection to identify NF-κB inhibitors and anti-inflammatory targets based on endothelial cell culture and network pharmacology. Journal of ethnopharmacology, 2015. 174: p. 270-276.
29. Kono, R., et al., Biological and epidemiological evidence of anti-allergic effects of traditional Japanese food ume (Prunus mume). Scientific reports, 2018. 8(1): p. 11638.
30. Yuan, C., et al., Procatechuic acid and protocatechuic aldehyde increase survival of Caenorhabditis elegans after fungal infection and inhibit fungal virulence. Frontiers in Pharmacology, 2024. 15: p. 1396733.
31. Wang, Y., et al., Antibacterial and antibiofilm activities of protocatechualdehyde and its synergy with ampicillin against methicillin-resistant Staphylococcus aureus. Frontiers in Microbiology, 2024. 15: p. 1366400.
32. Liu, Y. and L. Wang, Antibiofilm effect and mechanism of protocatechuic aldehyde against Vibrio parahaemolyticus. Frontiers in Microbiology, 2022. 13: p. 1060506.
33. Meng, X., et al., Inhibitory effect of protocatechualdehyde on Yersinia enterocolitica and its critical virulence factors. Microbial Pathogenesis, 2022. 173: p. 105877.
34. Kong, W.-J., et al., Combination of chemical fingerprint and bioactivity evaluation to explore the antibacterial components of Salvia miltiorrhizae. Scientific reports, 2017. 7(1): p. 8112.
35. Prachayasittikul, S., et al., Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules, 2008. 13(4): p. 904-921.
36. Chien, L.-H., et al., Study on the potential of Sanghuangporus sanghuang and its components as COVID-19 spike protein receptor binding domain inhibitors. Biomedicine & Pharmacotherapy, 2022. 153: p. 113434.
37. Zhou, Z., et al., Protocatechuic aldehyde inhibits hepatitis B virus replication both in vitro and in vivo. Antiviral research, 2007. 74(1): p. 59-64.
38. Zhang, Q.-Y., et al., Polyphenolic-modified cellulose acetate membrane for bone regeneration through immunomodulation. Carbohydrate Polymers, 2023. 305: p. 120546.
39. Du, Y., et al., Gelatin sponges with a uniform interoperable pore structure and biodegradability for liver injury hemostasis and tissue regeneration. Biomacromolecules, 2023. 24(11): p. 5313-5327.
40. He, S., et al., Differential action of pro-angiogenic and anti-angiogenic components of Danhong injection in ischemic vascular disease or tumor models. Chinese Medicine, 2022. 17(1): p. 4.
41. He, S., et al., A defined combination of four active principles from the Danhong injection is necessary and sufficient to accelerate EPC-mediated vascular repair and local angiogenesis. Frontiers in Pharmacology, 2019. 10: p. 1080.
42. Sun, S., et al., Protocatechualdehyde synergizes with aspirin at the platelet cyclooxygenase-1 level. Planta medica, 2011. 77(17): p. 1898-1904.
43. Liu, H., et al., Core bioactive components promoting blood circulation in the traditional Chinese medicine compound xueshuantong capsule (CXC) based on the relevance analysis between chemical HPLC fingerprint and in vivo biological effects. PloS one, 2014. 9(11): p. e112675.
44. Qin-Qin, Y., et al., Guanxinning tablet inhibits the interaction between leukocyte integrin Mac-1 and platelet GPIbα for antithrombosis without increased bleeding risk. Chinese Journal of Natural Medicines, 2022. 20(8): p. 589-600.
45. Liu, D., et al., RRP regulates autophagy through the AMPK pathway to alleviate the effect of cell senescence on atherosclerosis. Oxidative Medicine and Cellular Longevity, 2023. 2023(1): p. 9645789.
46. Zhang, L., et al., Ginsenoside Rg1-notoginsenoside R1-protocatechuic aldehyde reduces atherosclerosis and attenuates low-shear stress-induced vascular endothelial cell dysfunction. Frontiers in Pharmacology, 2021. 11: p. 588259.
47. Zhang, L., et al., Protocatechuic aldehyde increases pericyte coverage and mitigates pericyte damage to enhance the atherosclerotic plaque stability. Biomedicine & Pharmacotherapy, 2023. 168: p. 115742.
48. Kong, B.S., Y.H. Cho, and E.J. Lee, G protein-coupled estrogen receptor-1 is involved in the protective effect of protocatechuic aldehyde against endothelial dysfunction. PloS one, 2014. 9(11): p. e113242.
49. Xing, Y.L., et al., Protocatechuic aldehyde inhibits lipopolysaccharide‐induced human umbilical vein endothelial cell apoptosis via regulation of caspase‐3. Phytotherapy Research, 2012. 26(9): p. 1334-1341.
50. Cui, X., et al., Notoginsenoside R1-Protocatechuic aldehyde reduces vascular inflammation and calcification through increasing the release of nitric oxide to inhibit TGFβR1-YAP/TAZ pathway in vascular smooth muscle cells. International Immunopharmacology, 2024. 143: p. 113574.
51. Lin, B., et al., Lipid regulation of protocatechualdehyde and hydroxysafflor yellow A via AMPK/SREBP2/PCSK9/LDLR signaling pathway in hyperlipidemic zebrafish. Heliyon, 2024. 10(3).
52. Sun, M., et al., Composition, anti-LDL oxidation, and non-enzymatic glycosylation inhibitory activities of the flavonoids from Mesembryanthemum crystallinum. Frontiers in Nutrition, 2022. 9: p. 963858.
53. Zhou, J., et al., Pharmacokinetic study on protocatechuic aldehyde and hydroxysafflor yellow A of Danhong injection in rats with hyperlipidemia. Pharmacology, 2018. 102(3-4): p. 154-160.
54. Wu, R., et al., Salvia miltiorrhiza extract prevents the occurrence of early atherosclerosis in apoe-/-mice via TLR4/NF-kB pathway. Cardiovascular & Hematological Agents in Medicinal Chemistry, 2023. 21(3): p. 232.
55. Tang, Q., et al., A green and highly efficient method to deliver hydrophilic polyphenols of Salvia miltiorrhiza and Carthamus tinctorius for enhanced anti-atherosclerotic effect via metal-phenolic network. Colloids and Surfaces B: Biointerfaces, 2022. 215: p. 112511.
56. Bennett, M.R., S. Sinha, and G.K. Owens, Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res, 2016. 118(4): p. 692-702.
57. Moon, C.Y., et al., Protocatechuic aldehyde inhibits migration and proliferation of vascular smooth muscle cells and intravascular thrombosis. Biochemical and biophysical research communications, 2012. 423(1): p. 116-121.
58. Summerhill, V. and A. Orekhov, Pericytes in Atherosclerosis. Adv Exp Med Biol, 2019. 1147: p. 279-297.
59. Dabravolski, S.A., et al., Molecular mechanisms underlying pathological and therapeutic roles of pericytes in atherosclerosis. International Journal of Molecular Sciences, 2022. 23(19): p. 11663.
60. Wei, G., et al., Anti-inflammatory effect of protocatechuic aldehyde on myocardial ischemia/reperfusion injury in vivo and in vitro. Inflammation, 2013. 36: p. 592-602.
61. Jiang, L., et al., HIF-1α preconditioning potentiates antioxidant activity in ischemic injury: the role of sequential administration of dihydrotanshinone I and protocatechuic aldehyde in cardioprotection. Antioxidants & Redox Signaling, 2019. 31(3): p. 227-242.
62. Wu, X., et al., Protocatechuic aldehyde protects cardiomycoytes against ischemic injury via regulation of nuclear pyruvate kinase M2. Acta Pharmaceutica Sinica B, 2021. 11(11): p. 3553-3566.
63. Wan, Y.-J., et al., Protocatechualdehyde protects oxygen-glucose deprivation/reoxygenation-induced myocardial injury via inhibiting PERK/ATF6α/IRE1α pathway. European Journal of Pharmacology, 2021. 891: p. 173723.
64. Han, X.-D., et al., A computational and functional study elicits the ameliorating effect of the Chinese herbal formula Huo Luo Xiao Ling Dan on experimental ischemia-induced myocardial injury in rats via inhibition of apoptosis. Drug Design, Development and Therapy, 2015: p. 1063-1102.
65. Guo, X., et al., Thrombus-specific/responsive biomimetic nanomedicine for spatiotemporal thrombolysis and alleviation of myocardial ischemia/reperfusion injury. Journal of Nanobiotechnology, 2022. 20(1): p. 531.
66. Wang, P. and Q. Zhang, Protocatechuic aldehyde alleviates d-galactose–induced cardiomyocyte senescence by regulating the TCF3/ATG5 axis. Journal of Cardiovascular Pharmacology, 2023. 81(3): p. 221-231.
67. Wan, Y.-J., et al., Protocatechualdehyde reduces myocardial fibrosis by directly targeting conformational dynamics of collagen. European Journal of Pharmacology, 2019. 855: p. 183-191.
68. Fang, X., et al., Protocatechuic aldehyde protects against isoproterenol-induced cardiac hypertrophy via inhibition of the JAK2/STAT3 signaling pathway. Naunyn-Schmiedeberg's archives of pharmacology, 2018. 391: p. 1373-1385.
69. Zhang, J.-p., et al., Salvia miltiorrhiza (Danshen) injection ameliorates iron overload-induced cardiac damage in mice. Planta Medica, 2013. 79(09): p. 744-752.
70. He, F., et al., Protective Effect of Protocatechuic Aldehyde on Cerebral Ischemia/Reperfusion Injury in Rats through Blood—Brain Barrier Protection. Bulletin of Experimental Biology and Medicine, 2024. 177(6): p. 763-769.
71. Guo, Y., et al., Protocatechuic aldehyde prevents ischemic injury by attenuating brain microvascular endothelial cell pyroptosis via lncRNA Xist. Phytomedicine, 2022. 94: p. 153849.
72. Guo, C., et al., Protocatechualdehyde protects against cerebral ischemia-reperfusion-induced oxidative injury via protein kinase Cε/Nrf2/HO-1 pathway. Molecular neurobiology, 2017. 54: p. 833-845.
73. Kaya, Z.B., et al., Optimizing SH-SY5Y cell culture: exploring the beneficial effects of an alternative media supplement on cell proliferation and viability. Scientific Reports, 2024. 14(1): p. 4775.
74. Yuan, H., et al., Ligand fishing of monoamine oxidase B inhibitors from Platycodon grandiflorus (Jacq.) A. DC. roots by the enzyme functionalised magnetic nanoparticles. Phytochemical Analysis, 2023. 34(1): p. 67-75.
75. Guo, C., et al., Neuroprotective effects of protocatechuic aldehyde through PLK2/p-GSK3β/Nrf2 signaling pathway in both in vivo and in vitro models of Parkinson's disease. Aging (Albany NY), 2019. 11(21): p. 9424.
76. Zhao, X., et al., Neuroprotective effects of protocatechuic aldehyde against neurotoxin-induced cellular and animal models of Parkinson’s disease. PLoS One, 2013. 8(10): p. e78220.
77. Gao, J.-W., et al., DJ-1–Mediated Protective Effect of Protocatechuic Aldehyde Against Oxidative Stress in SH-SY5Y Cells. Journal of pharmacological sciences, 2011. 115(1): p. 36-44.
78. Gibson, R., S.P. Dalvi, and P.S. Dalvi, DJ-1 and Parkinson's disease. Brain Disorders, 2021. 3: p. 100020.
79. Zhao, Z., et al., Protocatechuic aldehyde promotes the functional recovery of spinal cord injury by activating the Wnt/β-catenin signaling pathway. The Journal of Spinal Cord Medicine, 2024. 47(5): p. 669-680.
80. Shi, X., et al., Protective effect of Gastrodia elata Blume in a Caenorhabditis elegans model of Alzheimer's disease based on network pharmacology. Biomedical Reports, 2023. 18(5): p. 1-12.
81. Wang, X., et al., Support for natural small-molecule phenols as anxiolytics. Molecules, 2017. 22(12): p. 2138.
82. Liang, Y., et al., Bioinspired injectable self-healing hydrogel sealant with fault-tolerant and repeated thermo-responsive adhesion for sutureless post-wound-closure and wound healing. Nano-Micro Letters, 2022. 14(1): p. 185.
83. Yang, M., et al., Green synthesis-inspired antibacterial, antioxidant and adhesive hydrogels with ultra-fast gelation and hemostasis for promoting infected skin wound healing. Acta Biomaterialia, 2024.
84. Zhou, S., et al., An Immune‐Regulating Polysaccharide Hybrid Hydrogel with Mild Photothermal Effect and Anti‐Inflammatory for Accelerating Infected Wound Healing. Advanced Healthcare Materials, 2024: p. 2400003.
85. Deng, J., et al., Antioxidant and antibacterial hydrogel formed by protocatechualdehyde–ferric iron complex and aminopolysaccharide for infected wound healing. International Journal of Biological Macromolecules, 2024. 268: p. 131642.
86. Zhang, Y., et al., Preparation of antibacterial hydrogel from poly (aspartic hydrazide) and quaternized N-[3-(dimethylamino) propyl] methylacrylamide copolymer with antioxidant and hemostatic effects for wound repairing. Colloids and Surfaces B: Biointerfaces, 2024. 238: p. 113881.
87. Bai, Y., et al., Metallic‐Polyphenolic Nanoparticles Reinforced Cationic Guar Gum Hydrogel for Effectively Treating Burn Wound. Macromolecular Bioscience, 2024. 24(3): p. 2300396.
88. Qiao, J., et al., Protocatechualdehyde-ferric iron tricomplex embedded gelatin hydrogel with adhesive, antioxidant and photothermal antibacterial capacities for infected wound healing promotion. International Journal of Biological Macromolecules, 2023. 242: p. 125029.
89. Yang, C., et al., Collagen-based hydrogels cross-linked via laccase-mediated system incorporated with Fe3+ for wound dressing. Colloids and Surfaces B: Biointerfaces, 2022. 219: p. 112825.
90. Liang, Y., et al., Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS nano, 2021. 15(4): p. 7078-7093.
91. Chen, F., et al., A Novel Multifunctional Crosslinking PVA/CMCS Hydrogel Containing Cyclic Peptide Actinomycin X2 and PA@ Fe with Excellent Antibacterial and Commendable Mechanical Properties. Chemistry & Biodiversity, 2023. 20(8): p. e202300831.
92. Zhang, W., et al., Antibacterial carboxymethyl chitosan hydrogel loaded with antioxidant cascade enzymatic system for immunoregulating the diabetic wound microenvironment. International Journal of Biological Macromolecules, 2024. 282: p. 137539.
93. Xiang, K., et al., Multifunctional ADM hydrogel containing endothelial cell-exosomes for diabetic wound healing. Materials Today Bio, 2023. 23: p. 100863.
94. Yang, Y., et al., M2 macrophage-polarized anti-inflammatory microneedle patch for accelerating biofilm-infected diabetic wound healing via modulating the insulin pathway. Journal of Nanobiotechnology, 2024. 22(1): p. 489.
95. Geng, X., et al., Preparation of ultra-small copper nanoparticles-loaded self-healing hydrogels with antibacterial, inflammation-suppressing and angiogenesis-enhancing properties for promoting diabetic wound healing. International Journal of Nanomedicine, 2023: p. 3339-3358.
96. Fu, Y.J., et al., All‐Natural Immunomodulatory Bioadhesive Hydrogel Promotes Angiogenesis and Diabetic Wound Healing by Regulating Macrophage Heterogeneity. Advanced Science, 2023. 10(13): p. 2206771.
97. Burgess, J.L., et al., Diabetic Wound-Healing Science. Medicina (Kaunas), 2021. 57(10).
98. Wang, Y., et al., Adhesive hydrogel releases protocatechualdehyde-Fe3+ complex to promote three healing stages for accelerated therapy of oral ulcers. Acta Biomaterialia, 2024. 178: p. 68-82.
99. Xia, Y., et al., Multifunctional Porous Bilayer Artificial Skin for Enhanced Wound Healing. ACS Applied Materials & Interfaces, 2024. 16(27): p. 34578-34590.
100. Wang, C., et al., Guanxining injection alleviates fibrosis in heart failure mice and regulates SLC7A11/GPX4 axis. Journal of Ethnopharmacology, 2023. 310: p. 116367.
101. Chang, Y.-T., et al., Anti-EMT and anti-fibrosis effects of protocatechuic aldehyde in renal proximal tubular cells and the unilateral ureteral obstruction animal model. Pharmaceutical Biology, 2022. 60(1): p. 1198-1206.
102. Du, X., et al., Tengdan capsule prevents hypertensive kidney damage in SHR by inhibiting periostin-mediated renal fibrosis. Frontiers in Pharmacology, 2021. 12: p. 638298.
103. Chang, Y.-T., et al., Evaluation of the therapeutic effects of protocatechuic aldehyde in diabetic nephropathy. Toxins, 2021. 13(8): p. 560.
104. Yang, J., et al., Protocatechualdehyde attenuates obstructive nephropathy through inhibiting lncRNA9884 induced inflammation. Phytotherapy Research, 2021. 35(3): p. 1521-1533.
105. Feng, W., Y. Ruchun, and T. Yuewen, Uncovering pharmacological mechanisms of Phellinus linteus on focal segmental glomeruloscleosis rats through tandem mass tag-based quantitative proteomic analysis, network pharmacology analysis and experimental validation. Journal of Traditional Chinese Medicine, 2023. 43(4): p. 744.
106. Gao, L., et al., Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing nox-mediated oxidative stress and renal inflammation. Frontiers in Pharmacology, 2016. 7: p. 479.
107. Li, J., et al., Improved dialysis removal of protein-bound uremic toxins by salvianolic acids. Phytomedicine, 2019. 57: p. 166-173.
108. Tao, R., et al., Cynomorium songaricum Rupr demonstrates phytoestrogenic or phytoandrogenic like activities that attenuates benign prostatic hyperplasia via regulating steroid 5-α-reductase. Journal of ethnopharmacology, 2019. 235: p. 65-74.
109. Huang, L.P., et al., A Modified Small Intestinal Submucosa Patch with Multifunction to Promote Scarless Repair and Reinvigoration of Urethra. Adv Healthc Mater, 2023. 12(23): p. e2300519.
110. Lee, Y.H., et al., Chemical screening identifies the anticancer properties of Polyporous parvovarius. Journal of Cancer, 2023. 14(1): p. 50.
111. Wang, Y., et al., Induction of apoptosis in CTLL-2 cells by protocatechualdehyde. Anticancer research, 2001. 21(2A): p. 1095-1101.
112. Nishimura, M., et al., Antiproliferative activity of protocatechualdehyde on Chinese hamster cells grown in culture. Gann= Gan, 1981. 72(6): p. 868-879.
113. Deng, Y., et al., Protocatechuic aldehyde represses proliferation and migration of breast cancer cells through targeting C-terminal binding protein 1. Journal of breast cancer, 2020. 23(1): p. 20-35.
114. Choi, J., et al., Anticancer activity of protocatechualdehyde in human breast cancer cells. Journal of medicinal food, 2014. 17(8): p. 842-848.
115. Lee, J.R., et al., The contribution of activating transcription factor 3 to apoptosis of human colorectal cancer cells by protocatechualdehyde, a naturally occurring phenolic compound. Archives of biochemistry and biophysics, 2014. 564: p. 203-210.
116. Jeong, J.B. and S.-H. Lee, Protocatechualdehyde possesses anti-cancer activity through downregulating cyclin D1 and HDAC2 in human colorectal cancer cells. Biochemical and Biophysical Research Communications, 2013. 430(1): p. 381-386.
117. Wang, N., et al., Identification of WT1 as determinant of heptatocellular carcinoma and its inhibition by Chinese herbal medicine Salvia chinensis Benth and its active ingredient protocatechualdehyde. Oncotarget, 2017. 8(62): p. 105848.
118. Ko, S.-C. and S.-H. Lee, Protocatechuic aldehyde inhibits α-MSH-induced melanogenesis in B16F10 melanoma cells via PKA/CREB-associated MITF downregulation. International Journal of Molecular Sciences, 2021. 22(8): p. 3861.
119. Pei, J., et al., Protocatechuic aldehyde acts synergistically with dacarbazine to augment DNA double-strand breaks and promote apoptosis in cutaneous melanoma cells. BMC complementary medicine and therapies, 2023. 23(1): p. 111.
120. Zhong, S., et al., Phellinus gilvus‑derived protocatechualdehyde induces G0/G1 phase arrest and apoptosis in murine B16‑F10 cells. Molecular medicine reports, 2020. 21(3): p. 1107-1114.
121. Ding, Y., C. Jiratchayamaethasakul, and S.-H. Lee, Protocatechuic aldehyde attenuates UVA-induced photoaging in human dermal fibroblast cells by suppressing MAPKs/AP-1 and NF-κB signaling pathways. International journal of molecular sciences, 2020. 21(13): p. 4619.
122. Hao, R., et al., Transdermal delivery of Protocatechuic aldehyde using hyaluronic acid/gelatin-based microneedles for the prevention and treatment of hypertrophic scars. European Journal of Pharmaceutics and Biopharmaceutics, 2023. 184: p. 202-213.
123. Xiong, X., et al., Qingxue jiedu formulation ameliorated DNFB-induced atopic dermatitis by inhibiting STAT3/MAPK/NF-κB signaling pathways. Journal of Ethnopharmacology, 2021. 270: p. 113773.
124. Kim, J.-E., et al., Effect of Ganoderma applanatum mycelium extract on the inhibition of adipogenesis in 3T3-L1 adipocytes. Journal of medicinal food, 2014. 17(10): p. 1086-1094.
125. Ma, R., H. Weng, and J. Liang, Screening of lipase inhibitors in Folium Mori with lipase‐linked magnetic microspheres by high‐performance liquid chromatography and evaluation in diabetic mice. Journal of separation science, 2016. 39(23): p. 4474-4483.
126. Zhang, J., et al., Mixed aqueous extract of Salvia miltiorrhiza reduces blood pressure through inhibition of vascular remodelling and oxidative stress in spontaneously hypertensive rats. Cellular Physiology and Biochemistry, 2016. 40(1-2): p. 347-360.
127. Jie, L., et al., Protocatechuic aldehyde attenuates chondrocyte senescence via the regulation of PTEN-induced kinase 1/Parkin-mediated mitochondrial autophagy. International Journal of Immunopathology and Pharmacology, 2024. 38: p. 03946320241271724.
128. Huang, J., et al., Cartilage Decellularized Matrix Hydrogel Loaded with Protocatechualdehyde for Targeted Epiphycan Treatment of Osteoarthritis. Materials Today Bio, 2024: p. 101124.
129. Steenkamp, W., et al., The correlation between clinical and radiological severity of osteoarthritis of the knee. SICOT-J, 2022. 8.
130. Li, Y., et al., Network pharmacology analysis and clinical verification of Jishe Qushi capsules in rheumatoid arthritis treatment. Medicine, 2023. 102(34): p. e34883.
131. Huang, H., et al., Protocatechualdehyde inhibits receptor activator of nuclear factor kappa‐B ligand‐induced osteoclastogenesis and attenuates lipopolysaccharide‐induced inflammatory osteolysis. Phytotherapy Research, 2021. 35(7): p. 3821-3835.
132. Tao, Z.-S., et al., Protocatechualdehyde inhibits iron overload-induced bone loss by inhibiting inflammation and oxidative stress in senile rats. International Immunopharmacology, 2024. 141: p. 113016.
133. Li, H., et al., Anti-gout effects of the medicinal fungus phellinus igniarius in hyperuricaemia and acute gouty arthritis rat models. Frontiers in Pharmacology, 2022. 12: p. 801910.
134. Wang, Y.-H., et al., Protocatechualdehyde prevents methylglyoxal-induced mitochondrial dysfunction and AGEs-RAGE axis activation in human lens epithelial cells. European journal of pharmacology, 2014. 738: p. 374-383.
135. Kim, Y.S., et al., Effect of protocatechualdehyde on receptor for advanced glycation end products and TGF-β1 expression in human lens epithelial cells cultured under diabetic conditions and on lens opacity in streptozotocin-diabetic rats. European journal of pharmacology, 2007. 569(3): p. 171-179.
136. Byun, J.W., et al., Therapeutic effect of protocatechuic aldehyde in an in vitro model of Graves' orbitopathy. Investigative Ophthalmology & Visual Science, 2016. 57(10): p. 4055-4062.
137. Xu, Y., et al., Protocatechuic aldehyde protects against experimental sepsis in vitro and in vivo. Basic & clinical pharmacology & toxicology, 2012. 110(4): p. 384-389.
138. Jiang, M., et al., Identification of NF-κB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. Journal of Ethnopharmacology, 2013. 147(2): p. 426-433.
139. Cao, H., et al., Robust and Multifunctional Therapeutic Nanoparticles against Peritonitis-Induced Sepsis. Biomacromolecules, 2024. 25(2): p. 1133-1143.
140. Jang, B.-S., et al., Extracts of Phellinus gilvus and Phellinus baumii inhibit pulmonary inflammation induced by lipopolysaccharide in rats. Biotechnology Letters, 2004. 26: p. 31-33.
141. Zhang, J., et al., Lung morphometry changes in prevention of airway remodeling by protocatechuic aldehyde in asthmatic mice. International Journal of Clinical and Experimental Medicine, 2015. 8(5): p. 6890.
142. Zhang, L., et al., Protocatechuic aldehyde ameliorates experimental pulmonary fibrosis by modulating HMGB1/RAGE pathway. Toxicology and Applied Pharmacology, 2015. 283(1): p. 50-56.
143. Yin, J., et al., The acute toxicity and cardiotoxic effects of protocatechuic aldehyde on Juvenile Zebrafish. Toxics, 2024. 12(11): p. 799.
144. Liu, Y.R., et al., The effect of the major components of Salvia miltiorrhiza Bunge on bone marrow cells. J Ethnopharmacol, 2007. 111(3): p. 573-83.
Downloads
Published
Versions
- 2026-01-12 (3)
- 2026-01-19 (2)
- 2026-01-12 (1)
Data Availability Statement
All data supporting this study are contained within the article.
Issue
Section
License
Copyright (c) 2026 Bahareh Hasan pour, Roya Hasan pour (Author)

This work is licensed under a Creative Commons Attribution 4.0 International License.